Highly enantioselective tandem enzyme–organocatalyst crossed aldol reactions with acetaldehyde in deep-eutectic-solvents†
Abstract
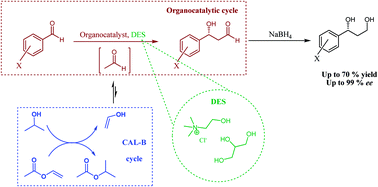
Deep-eutectic-solvents (DES), e.g. choline chloride and glycerol, have emerged as promising bio-based and cost-effective reaction media. Herein, the first concept of tandem catalysis of enzymes and organocatalysts in such environmentally-friendly DES is reported, focusing on enzymatic in situ acetaldehyde production combined with highly enantioselective crossed aldehyde–aldehyde C–C bond formation organocatalysis. This leads to an integrative concept with straightforward product recovery and promising catalyst recycling, enabling the synthesis of highly valuable optically active building blocks under mild reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...