s-Tetrazines functionalized with phenols: synthesis and physico-chemical properties†
Abstract
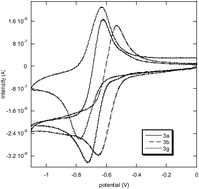
s-Tetrazines functionalized with phenols substituted by electron withdrawing or electron donating groups have been synthesized. The electrochemical and photophysical properties of these series of compounds have been investigated. All the new tetrazines prepared can be reversibly reduced into their anion-radicals, and the reduction potential of the tetrazine is sensitive (ΔE of 0.1–0.2 V) to the meta- and the para-substitution of the phenoxy group in its 6-position. Whereas tetrazines substituted by phenoxy possessing an electron donating moiety are virtually non emissive, the ones substituted by a phenoxy possessing an electron withdrawing group are fluorescent compounds (quantum yield up to 0.36). Fluorescence quenching experiments have been performed on all the fluorescent compounds. A moderate fluorescence quenching is observed in the presence of toluene, m-xylene and styrene, whereas an efficient quenching occurs in the presence of bisphenol A, in agreement with quenching through a photoinduced electron transfer, which depends on the electron-rich character of the quencher.

 Please wait while we load your content...
Please wait while we load your content...