Probing the effect of electron donation on CO2 absorbing 1,2,3-triazolide ionic liquids†
Abstract
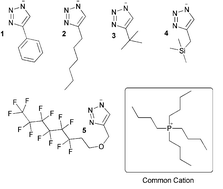
Development of the next generation materials for effective separation of gases is required to address various issues in energy and environmental applications. Ionic liquids (ILs) are among the most promising material types. To overcome the many hurdles in making a new class of materials technologically applicable, it is necessary to identify, access, and scale up a range of representative substances. In this work, CO2 reactive triazolide ILs were synthesized and characterized with the aim of developing a deeper understanding of how structural changes affect the overall properties of these substances. It was found that substituents on the anion play a crucial role in dictating the physical properties for CO2 capture. Depending upon the anion substituent, CO2 capacities between 0.07 and 0.4 mol CO2 per mol IL were observed. It was found that less sterically-hindered anions and anions containing electron donating groups were more reactive towards CO2. Detailed spectroscopic, CO2 absorption, rheological, and simulation studies were carried out to understand the nature and influence of these substituents. The effect of water content was also evaluated, and it was found that water had an unexpected impact on the properties of these materials, resulting in an increased viscosity, but little change in the CO2 reactivity.

 Please wait while we load your content...
Please wait while we load your content...