Polysubstituted pyrrole derivatives via 1,2-alkenyl migration of novel γ-amino-α,β-unsaturated aldehydes and α-diazocarbonyls†
Abstract
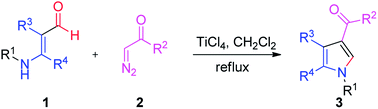
Tri-substituted pyrrole derivatives were successfully synthesized from aminoaldehyde and α-diazocarbonyl, using a TiCl4 catalyst. The reactions proceeded via 1,2-migration and condensation, leading to the corresponding pyrroles in moderate to excellent yields. Reaction susceptibility was subsequently demonstrated by the variation in substrates.

 Please wait while we load your content...
Please wait while we load your content...