Hybrid assembly of DNA-coated gold nanoparticles with water soluble conjugated polymers for studying protein–DNA interaction and ligand inhibition†
Abstract
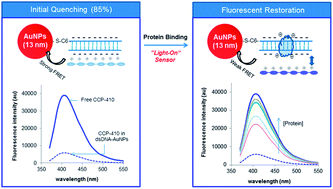
Metal nanoparticles (mNPs) have unique optical properties arising from the localized surface plasmon resonance. They have been extensively used as colorimetric probes for chemical and biological analysis, exploiting particle aggregation-induced color change. They can also support fluorimetric detection based on Förster resonance energy transfer (FRET) or nanoparticle surface energy transfer (NSET) with proximal fluorophores. In this paper, luminescent water soluble conjugated polymers (CPs) and dsDNA-coated gold NPs (AuNPs) are used as collaborative sensing elements for studying protein–dsDNA interactions. The hybrid materials-based assays exploit the phenomena of (1) CPs' fluorescence emission can be quenched by AuNPs due to CPs–DNA interactions on AuNPs surface and (2) protein binding to DNA can change the surface charge of the dsDNA–AuNPs conjugates that in turn change the degree of CPs quenching. Three CPs of bearing with different charge and emitting at different wavelength are used to construct the hybrid sensors for determining protein–DNA interactions in terms of sequence selectivity, binding affinity and binding stoichiometry. Depending on the initial quenching, determined by CPs' charge property and emission wavelength relative to the absorption peak of 13 nm AuNPs, “light-on”, “light-off”, and “two way” assays have been constructed that are capable of studying proteins of known or unknown charge properties. We have demonstrated the concept for two important oncogenic factors, i.e. FoxA1 (forkhead boxA1) and AP-2γ (activating enhancer binding protein 2 gamma). They are pivotal in regulating the transcriptional activity of estrogen receptor alpha and controlling the expression of estrogen-responsive breast cancer cells. Determination of their DNA binding properties can reveal how they regulate the transcriptional activity of estrogen receptor. The hybrid sensors have been extended to screen small molecular weight ligands that can inhibit FoxA1– and AP-2γ–DNA complex formation. Identification of inhibitors as drug candidates targeting these two transcription factors could be an alternative in treating breast cancer, in particular those that have become endocrine resistant.

 Please wait while we load your content...
Please wait while we load your content...