Structure and thermal properties of composites with RE-borohydrides (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Er, Yb or Lu) and LiBH4†
Abstract
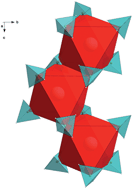
RECl3 (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Er, Yb, Lu) and LiBH4 ball-milled in the molar ratio of 1 : 6 resulted in formation of numerous rare-earth metal (RE) borohydrides and LiCl in mixtures with excess LiBH4. The reaction products were characterized by powder X-ray diffraction and infrared spectroscopy, and their hydrogen sorption properties were investigated by thermogravimetric analysis combined with differential scanning calorimetry and in situ synchrotron radiation powder X-ray diffraction. La, Ce, Pr and Nd form LiRE(BH4)3Cl compounds which crystallize in the cubic space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m. Sm, Gd, Tb, Er and Yb form RE(BH4)3 phases crystallizing in the cubic space group Pa
3m. Sm, Gd, Tb, Er and Yb form RE(BH4)3 phases crystallizing in the cubic space group Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , with a possible polymorphic transition to a higher symmetry space group, Pm
, with a possible polymorphic transition to a higher symmetry space group, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The smaller RE-elements Yb and Lu form tetrahedral [RE(BH4)4]− anionic complexes stabilized by Li+ cations crystallizing in the tetragonal space group P
m. The smaller RE-elements Yb and Lu form tetrahedral [RE(BH4)4]− anionic complexes stabilized by Li+ cations crystallizing in the tetragonal space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2c. Additionally Sm and Gd also exhibit a transition to the LiRE(BH4)3Cl phases observed for the largest lanthanides. LiSm(BH4)3Cl is reduced to Sm(BH4)2 upon heating, which takes a previously unreported orthorhombic structure in space group Pbcn. Eu did not form any of these RE-borohydride compounds, but infrared spectroscopy indicates formation of a new borohydride phase. Hydrogen desorption from the composite mixtures starts around 200 °C. Rehydrogenation of the La-system under p(H2) = 10 MPa at 300 °C showed a partial reversibility, with 18% recovery of the original hydrogen capacity, which increased to 36% at 415 °C. Rehydrogenation under p(H2) = 10 MPa at 400 °C showed a partial reversibility of 25% in the Er-system. Formation of REH2 during thermal decomposition destabilizes the remaining LiBH4 in the composites, thus releasing hydrogen at lower temperatures than for pure LiBH4.
2c. Additionally Sm and Gd also exhibit a transition to the LiRE(BH4)3Cl phases observed for the largest lanthanides. LiSm(BH4)3Cl is reduced to Sm(BH4)2 upon heating, which takes a previously unreported orthorhombic structure in space group Pbcn. Eu did not form any of these RE-borohydride compounds, but infrared spectroscopy indicates formation of a new borohydride phase. Hydrogen desorption from the composite mixtures starts around 200 °C. Rehydrogenation of the La-system under p(H2) = 10 MPa at 300 °C showed a partial reversibility, with 18% recovery of the original hydrogen capacity, which increased to 36% at 415 °C. Rehydrogenation under p(H2) = 10 MPa at 400 °C showed a partial reversibility of 25% in the Er-system. Formation of REH2 during thermal decomposition destabilizes the remaining LiBH4 in the composites, thus releasing hydrogen at lower temperatures than for pure LiBH4.

 Please wait while we load your content...
Please wait while we load your content...