Green chemical incorporation of silicon into polyoxoanions of molybdenum: characterization, thermal kinetics study and their photocatalytic water splitting activity†
Abstract
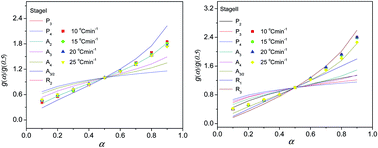
Cetylpyridinium silicomolybdate (CSM) nanorods were successfully synthesized by applying green chemistry principles using sodium molybdate and a structure directing cationic surfactant, cetyl pyridinium chloride (CPC) at room temperature. The composition and morphology of the nanorods were established by Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), thermogravimetric analysis (TG) and inductively coupled plasma atomic emission spectroscopic (ICP-AES) techniques. The thermal decomposition kinetics of CSM nanorods were investigated by a non-isothermal thermogravimetric analyzer at various heating rates. The thermal decomposition of CSM occurred in two stages. The activation energies of the first and second stages of thermal decomposition for all heating rates have been estimated using the iso-conventional methods of Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS) and the results are found to be in good agreement with each other. The invariant kinetic parameter (IKP) method and master plot method were also used to evaluate the kinetic parameters and mechanism for the thermal decomposition of CSM. The photocatalytic water oxidation mechanism using the CSM catalyst in the presence of platinum (Pt) co-catalyst enhances the H2 evolution and was found to be 1.946 mmol g−1 h−1.

 Please wait while we load your content...
Please wait while we load your content...