Investigation of stainless steel pickling liquor as a precursor for high Capacity battery electrode materials
Abstract
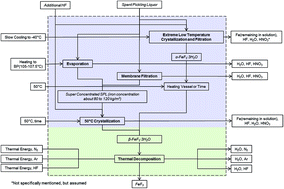
Stainless steel and battery manufacturing industries can be united for mutual economic and environmental benefits by utilizing stainless steel pickling liquor waste product as a precursor for high energy iron fluoride based positive electrode materials in batteries. This study analyzes the feasibility, environmental, and economic cost of the ferric fluoride (FeF3) synthesis approach through the use of recycled pickling liquor from the stainless steel fabrication industry. This new synthesis method is determined as more environmentally friendly than the current methods of disposing spent stainless steel pickling liquor and producing ferric fluoride separately. X-ray diffraction analysis demonstrated the synthesis occurs in two steps: the conversion of spent pickling liquor to produce the crystallohydrate β-FeF3·3H2O followed by its dehydration into anhydrous FeF3. Materials obtained from pickling solutions were found electrochemically active with improved cycling stability and similar capacity compared to commercial FeF3. Pickling solutions synthesized with nickel and chromium to better replicate stainless steel industry spent pickling waste showed improved electrochemical performance.

 Please wait while we load your content...
Please wait while we load your content...