Stereoselective adsorption utilizing l-phenylalanine imprinting chiral ordered mesoporous silica†
Abstract
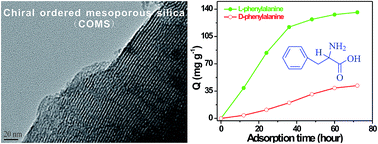
L-Phenylalanine imprinting chiral ordered mesoporous silica (L-Phe-COMS) was facilely synthesized in the presence of phenylalanine amino acid by combining tetraethyl orthosilicate and quaternized aminosilane silica sources. The obtained COMS was comparable with a MCM-41-type structure, with narrow pore size distribution, and high specific surface area characterized by powder X-ray diffraction and N2 adsorption experiments. The imprinting chirality of COMS was disclosed by mixed and separate L- and D-phenylalanine adsorption on the L-Phe-COMS with a stereoselective adsorption capacity of up to 3.24. In addition, six racemic mixtures including amino acids and drugs were explored to test the stereoselective adsorption capacity of L-Phe-COMS. The imprinting chiral ordered mesoporous silica presents the advantages of straightforward synthesis approach and robust stereoselective adsorption capacity, making it a promising candidate for chiral adsorption and separation.

 Please wait while we load your content...
Please wait while we load your content...