Iron-catalyzed C(sp3)–H functionalization of methyl azaarenes: a green approach to azaarene-substituted α- or β-hydroxy carboxylic derivatives and 2-alkenylazaarenes†
Abstract
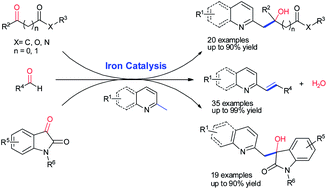
Bioactive azaarene-substituted lactic acids, β-hydroxy esters, 3-hydroxy-2H-indol-2-ones, and 2-alkenylazaarenes were prepared in moderate-to-excellent yields via C(sp3)–H functionalization of methyl azaarenes with carbonyl compounds in the presence of iron(II) acetate as an inexpensive, nontoxic, efficient catalyst. The application of this atom-, step-economic, and environmentally friendly method was demonstrated by a gram-scale synthesis of 3-[(E)-2-(7-chloroquinolin-2-yl)vinyl]benzaldehyde, a key intermediate of leukotriene receptor antagonist (Montelukast).

 Please wait while we load your content...
Please wait while we load your content...