Potential anti-bacterial agents: montmorillonite clay-catalyzed synthesis of novel 2-(3,5-substituted-1H-pyrazol-1-yl)-3-substituted quinolines and their in silico molecular docking studies†
Abstract
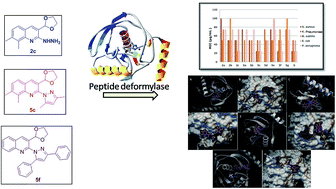
A series of 2-(3,5-substituted-1H-pyrazol-1-yl)-3-substituted quinolines 5a–g, were obtained from 2-chloro-3-(1,3-dioxolan-2-yl) quinolines 2a–c and diketones 3a–c with great yields by utilizing Montmorillonite clay K-10 as a catalyst in an eco-friendly methodology. All the synthesized compounds were characterized by FTIR, 1H-NMR, 13C-NMR and HRMS spectral techniques. Antibacterial screening of the compounds revealed that some of the compounds demonstrate moderate to good results. Amongst all, compound 2c displayed a good inhibitory profile against P. aeruginosa and B. subtilis, while compound 5c exhibited remarkable activity against S. aureus, B. subtilis and P. aeruginosa with an MIC value of 25 μg mL−1. Moreover, compound 5c presented a MIC value of 50 μg mL−1 against E. coli and K. pneumoniae, which was comparable to that of the standard. In addition, compound 5f was demonstrated good viability against S. aureus and P. aeruginosa with a MIC value of 25 μg mL−1. Compounds 2c and 5f, however, showed relatively moderate inhibition against S. aureus, while the latter had a moderate activity against B. subtilis. To further demonstrate the antibacterial efficacy of 2c, 5c and 5f, molecular docking studies were done, confirming that compound 5c possesses good binding interaction with the peptide deformylase protein, with a binding energy of −8.1 kcal mol−1—more prominent than that of standard ampicillin (−7.5 kcal mol−1)—while compounds 2c and 5f have moderate binding affinity.

 Please wait while we load your content...
Please wait while we load your content...