Solvent-free mechanochemical route for green synthesis of pharmaceutically attractive phenol-hydrazones†
Abstract
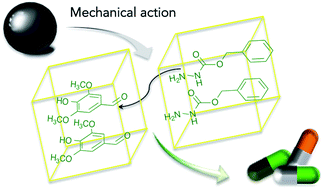
A series of hydrazones, as potential therapeutic agents, were successfully synthesized in a vibratory ball-mill from various substituted organic hydrazines and phenol aldehydes. The degree of conversion was increased by high electronic density on the amino group of the hydrazine reactant, as well as low steric hindrance around both reactive sites. In this particular case, the flexibility of the chain bearing the amino reactive site of hydrazine was highlighted as a factor influencing the reaction rate. The results showed that hydrazones could be obtained with more than a 99% transformation, without concurring by-products. This is highly adapted to the synthesis of active pharmaceutical ingredients, requiring a high level of purity. Owing to the fact that neither an environmentally unadvisable reagent nor additives or catalysts were added to achieve the transformation, this synthesis provides a good example and a prefiguration of an efficient green pharmaceutical process.

 Please wait while we load your content...
Please wait while we load your content...