Development of regioselective deacylation of peracetylated β-d-monosaccharides using lipase from Pseudomonas stutzeri under sustainable conditions†
Abstract
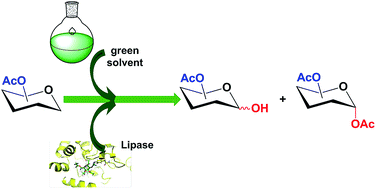
The lipase-catalyzed regioselective deacylation of peracetylated pyranosides has been evaluated in biosolvents. Among the biocatalysts tested, lipase from Pseudomonas stutzeri showed the highest activity, displaying regiospecific activity towards the anomeric position. This lipase was also employed in the regioselective alcoholysis of peracetylated sugars in green solvents, contributing to improve the sustainability of the process. Yields up to 97% of the desired product with different biosolvents were found. These reactions took place without noticeable activity and with total regioselectivity, representing a considerable improvement over the use of an aqueous buffer or conventional organic solvents. Furthermore, scaled up reactions are feasible without losing catalytic action. In order to understand the role of these biosolvents in the enzyme's synthetic behaviour, molecular modelling and docking studies were performed in the presence of some selected biosolvents to conclude that the presence of biosolvents in the reaction media modifies the access of the alcohols to the enzymatic active site allowing the presence of small alcohols and not i-propyl and t-butyl residues in the alcohol.

 Please wait while we load your content...
Please wait while we load your content...