Regioselective one-pot, three-component synthesis of substituted 2H-indazoles from 2-nitroarylaldehyde, alkyne and amine catalyzed by the CuBr/Zn(OTf)2 system†
Abstract
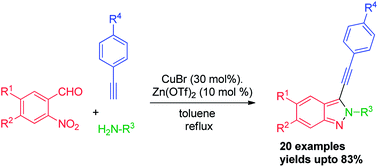
3-(Arylethynyl)-2H-indazoles can be effectively synthesized in one-pot using 2-nitroarylaldehydes, primary amines and alkynes co-catalyzed by copper(I) bromide and zinc(II) triflate. This method has a broad substrate scope with high to medium tolerance for a variety of functional groups.

 Please wait while we load your content...
Please wait while we load your content...