Treatment of acetamiprid insecticide from artificially contaminated water by colloidal manganese dioxide in the absence and presence of surfactants
Abstract
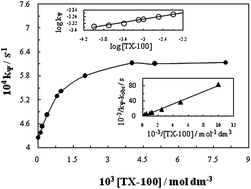
Acetamiprid is one of the most important pesticides and is effective against a number of insects. The increasing use of insecticides in the agricultural field is associated with a significant risk to water resources and aquatic systems. Thus the degradation of such compounds, after fulfillment of their insecticidal role, is essential to eliminate or minimize the contamination of water. The degradative treatment of acetamiprid insecticide from artificially contaminated water by water soluble colloidal MnO2 in acidic medium (HClO4) has been studied spectrophotometrically in the absence and presence of surfactants. The experiments have been performed under the pseudo-first-order reaction conditions with respect to MnO2. The degradation kinetics has been observed to be first-order with respect to MnO2 while fractional-order in both acetamiprid and HClO4. The anionic surfactant, sodium dodecyl sulfate (SDS) has been observed to be ineffective. On the other hand the reaction in the presence of cationic surfactant, cetyltrimethyl ammonium bromide (CTAB) could not be followed as well because it possesses a positive charge opposite to that of colloidal MnO2 causing flocculation and therefore could not be studied further. However, the addition of non-ionic surfactant, polyethylene glycol tert-octylphenyl ether (TX-100) accelerates the reaction rate. The catalytic effect of TX-100 has been discussed in the light of the available mathematical model. The kinetic data have been exploited to generate the various activation parameters for the oxidative degradation of acetamiprid by colloidal MnO2 in the absence and presence of non-ionic surfactant, TX-100.

 Please wait while we load your content...
Please wait while we load your content...