Rh(iii)-catalyzed annulation of N-methoxybenzamides with ynesulfonamides at room temperature: a practical and efficient route to 4-aminoisoquinolone derivatives†
Abstract
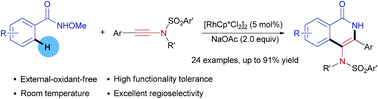
A practical and efficient Rh(III)-catalyzed annulation of N-methoxybenzamides with ynesulfonamides for the synthesis of 4-aminoisoquinolone derivatives has been developed under external-oxidant-free conditions at room temperature. This protocol features good functional group tolerance and excellent selectivity.

 Please wait while we load your content...
Please wait while we load your content...