Highly efficient synthesis and antioxidant capacity of N-substituted benzisoselenazol-3(2H)-ones†
Abstract
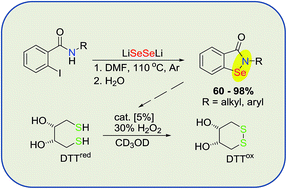
A new, general one step synthesis of N-substituted benzisoselenazol-3(2H)-ones based on the reaction of o-iodobenzamides with lithium diselenide, is described. A series of alkyl and aryl derivatives was obtained in high yields (up to 98%). Their GPx-like antioxidant activity, tested by NMR, showed a significantly higher activity than ebselen.

 Please wait while we load your content...
Please wait while we load your content...