Metal-free DDQ-mediated oxidative C–O coupling of acetalic sp3 C–H bonds with carboxylic acids†
Abstract
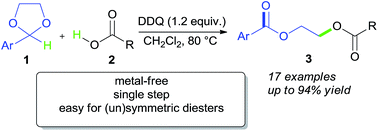
A simple and efficient DDQ-mediated oxidative C–O coupling reaction between acetalic sp3 C–H and carboxylic acid O–H bonds has been developed. This novel transformation smoothly proceeds without any metal catalyst and allows for the highly efficient synthesis of (un)symmetric glycol diesters in a single step.

 Please wait while we load your content...
Please wait while we load your content...