Design and synthesis of galactose-6-OH-modified α-galactosyl ceramide analogues with Th2-biased immune responses†
Abstract
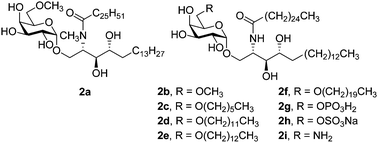
In this study, a simple type of O-6 analogue of KRN7000 was synthesized starting from galactosyl iodide and D-lyxose. This transformation involved the formation of a key disaccharide, the Wittig olefination on the anomeric hemiketal with simultaneous opening of the furanose ring, and azido substitution of the revealed OH group, Staudinger reaction, and an amide bond formation with global deprotection, which furnished various O-6 substituted analogues of KRN7000. Studies of immune modulating effects of these compounds on human dendritic cells and NKT cells revealed that longer acyl chain at Gal 6′ of α-GalCer induced more interleukin-4 with greater IL-4/IFN-γ ratios. These new analogues may have potential applications in the field of vaccine adjuvants and Th1-dominated autoimmune disorders by skewing the immune response of CD1d reactive NKT cell toward Th2. On the other hand, modification of 6′-OH of galactose with amine might induce stronger Th1 immune response than α-GalCer. Thus, modification of 6′-OH of galactose could regulate NKT cells to modulate the immune system toward Th1 or Th2 responses.

 Please wait while we load your content...
Please wait while we load your content...