Synthesis and antibacterial evaluation of novel Schiff's base derivatives of nitroimidazole nuclei as potent E. coli FabH inhibitors†
Abstract
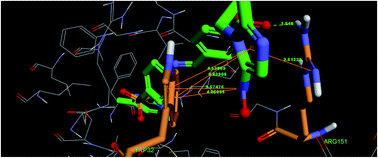
Series of novel Schiff's base derivatives have been synthesized by combining 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl 4-formylbenzoate 5, 6 with aromatic/heterocyclic amine 7a–r, 8, 9a–r in ethanol. All compounds were evaluated for antibacterial assay and inhibition against E. coli FabH. Among the compounds studied, most of the compounds showed effective antibacterial and potential inhibitory activity against E. coli FabH. Compound 10q showed most potent inhibitory activity (IC50 = 2.6883 μM) by binding tightly to the active site of the E. coli FabH receptor with minimum binding energy (ΔGb = −55.3117 kcal mol−1), in which molecular docking study indicated the binding mode was stabilized by one hydrogen bond and five π–π interactions.

 Please wait while we load your content...
Please wait while we load your content...