Synthesis of ganglioside Hp-s1†
Abstract
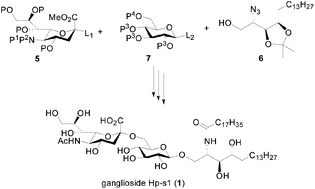
A simple protocol for the synthesis of a ganglioside Hp-s1 (1) starting from commercially available phytosphingosine, sialic acid, and D-glucose is described. This synthesis involved a glycosylation reaction of a phytosphingosine derived acceptor with a highly active benzyl protected glucosyl donor, a second glycosylation of a glucosyl acceptor with a sialyl donor followed by Staudinger reaction, amidation, and global deprotection as key steps.

 Please wait while we load your content...
Please wait while we load your content...