A convenient method for producing mono- and dichlorohydrins from glycerol
Abstract
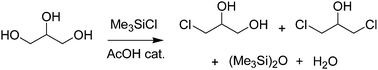
A new method for the transformation of glycerol into mono- and dichlorohydrins has been studied. With trimethylchlorosilane as chlorinating agent and acetic acid as catalyst, mono- and dichlorohydrins have been obtained in high yields and selectivity. In fact, under different reaction conditions, the synthesis of α-monochlorohydrin (3-chloropropan-1,2-diol) or α,γ-dichlorohydrin (1,3-dichloropropan-2-ol) as predominant product has been achieved. This process was also exploited for the valorisation of the crude mixture of glycerol and monochlorohydrin (glyceric mixture), a by-product from an earlier BioDiesel production. A reaction mechanism has been proposed based on investigations on the chlorination of different alcohols.

 Please wait while we load your content...
Please wait while we load your content...