High electrocatalytic activity of W18O49 nanowires for cobalt complex and ferrocenium redox mediators†
Abstract
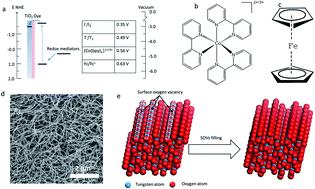
Understanding the relationship between the surface of electrocatalysts and the catalytic properties of different redox mediators is beneficial to the rational design of efficient catalysts for use in practical catalytic processes. Previous research observed that surface oxygen vacancies (SOVs) affected the catalytic activity for triiodide/iodide (I−/I3−) and T2/T− (T− = 5-mercapto-1-methyltetrazole ion) redox mediators in dye-sensitized solar cells (DSCs). However, the electrocatalytic properties of larger and steric metal complex redox mediators (cobalt complex, ferrocenium) on SOVs of W18O49 are unclear and have never been reported. In this study, we investigated the electrocatalytic properties of cobalt complex and ferrocenium redox mediators on SOVs of W18O49. Results indicated that the catalytic performance of W18O49 nanowires (NWs) as a counter electrode for cobalt complex and ferrocenium redox mediators was comparable to that of Pt. After SOVs filling, the reduction reaction activity of the cobalt complex decreases slightly whereas it increases slightly for ferrocenium. These findings enrich our understanding of heterogeneous catalytic reactions on the surface of transition metal complexes for different redox mediators.

 Please wait while we load your content...
Please wait while we load your content...