Interactions of multilayer ethanol, acetone, and diethyl ether films with clean and oxygenated vanadium substrates
Abstract
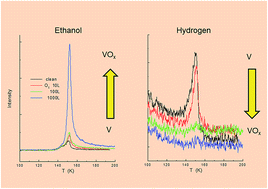
Thin films of ethanol, acetone, and diethyl ether were deposited onto a polycrystalline vanadium substrate, and thermal desorption and interfacial reaction of molecules were investigated using temperature programmed desorption and time-of-flight secondary ion mass spectrometry. Most of the molecules in the multilayer films are decomposed on a thoroughly deoxygenated V substrate, and reaction products other than hydrogen are fundamentally incorporated in subsurface sites. The hydrogen is liberated at the thermal desorption temperature of ethanol because of the O–H bond scission whereas acetone and diethyl ether are dehydrogenated rather gradually because the C–H bond is broken slowly after alkyl fragments are incorporated in subsurface sites. A small amount of ethylene (water) desorbs from ethanol on the deoxygenated (oxygenated) V substrate. A considerably large amount of oxygen is required for inactivation of the deoxygenated V substrate because oxides are formed in subsurface layers.

 Please wait while we load your content...
Please wait while we load your content...