Retracted Article: Equilibrium and kinetic studies on adsorptive removal of malachite green by the citrate-stabilized magnetite nanoparticles
Abstract
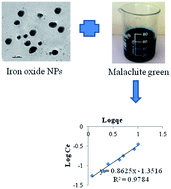
The present study investigated the adsorptive removal of malachite green (MG) from aqueous solution by using the citrate-stabilized magnetite nanoparticles (NPs). Magnetite NPs were synthesized chemically by precipitation method and they were characterized by XRD, TEM, particle size analyzer and zeta potential measurement. The mean diameter and zeta potential of the NPs was determined to be 25 nm and −33.14 mV, respectively. The adsorption of MG on the magnetite NPs was fitted better to Freundlich than Langmuir model. Kinetics of adsorption follows pseudo-second order rather than pseudo-first-order kinetic model. The pH of the interaction medium influenced the adsorption process. The adsorption of MG on the iron oxide NPs was decreased upon the increase of pH values. The rate of adsorption decreased with the increase of NaCl concentration in interaction medium. The present study may be applied for large scale to remove the dye stuffs from industrial effluents.

 Please wait while we load your content...
Please wait while we load your content...