A highly selective and sensitive fluorescent chemosensor for Hg2+ based on a pyridine-appended π-conjugated ligand†
Abstract
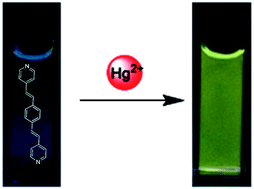
A pyridine-appended π-conjugated ligand 1,4-bis[2-(4-pyridyl)ethenyl]benzene (bpeb) has proved to be highly selective and sensitive as a chemosensor for Hg2+ in buffered aqueous solution. Remarkable changes in the UV-vis and fluorescence emission spectra were observed upon addition of Hg2+ ions to a solution of bpeb. In particular, the interaction of the lone-pair electrons on the pyridine nitrogen with Hg2+ ions results in a significant red-shift in the fluorescence spectrum and a large Stokes shift of up to 65 nm. DFT calculations have revealed that the energy levels of the HOMO and LUMO of bpeb are critically decreased upon coordination to Hg2+ ions, thereby accounting for this red-shift of the fluorescence spectrum. Furthermore, bpeb has been shown to be applicable as a fluorescent probe for imaging Hg2+ ions in PC3 cell lines, which may help in the understanding of relevant biological processes at the molecular level.

 Please wait while we load your content...
Please wait while we load your content...