Enhancing the lithiation rate of silicon nanowires by the inclusion of tin†
Abstract
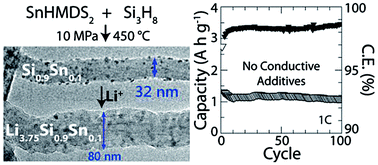
Silicon (Si) has a very high lithium storage capacity and is being explored as a negative electrode material in lithium-ion batteries (LIBs). Si nanowires can exhibit relatively stable performance for many cycles of charging; however, conductive carbon must often be added to the electrode layer to improve the rate capability due to the relatively low electrical conductivity of Si. The added carbon lowers the capacity of the electrode. Here, we show that the rate capability of Si in LIBs can be substantially enhanced by incorporating tin (Sn) into Si nanowires. The solubility of Sn in Si is very low (0.015 at%); yet, Sn used as a seed for supercritical fluid–liquid–solid (SFLS) growth can be trapped in Si nanowires with relatively high concentration (10 at%). Such Sn-containing Si nanowires and no added conductive carbon in the electrode layer, could be cycled in LIBs with high capacity (∼1000 mA h g−1 over 100 cycles) at a current density of 2.8 A g−1 (1 C). Capacities exceeding that of graphite could still be reached at cycle rates as high as 2 C. Real-time in situ transmission electron microscopy (TEM) revealed that lithiation occurs five times faster in Si nanowires with significant amounts of Sn than in the Si nanowires without Sn, and twice as fast as in nanowires that were coated with carbon.
- This article is part of the themed collection: Materials for Energy storage

 Please wait while we load your content...
Please wait while we load your content...