Reversal of the enantioselectivity in aldol addition over immobilized di- and tripeptides: studies under continuous flow conditions†
Abstract
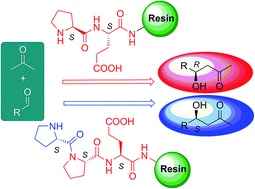
Heterogeneous asymmetric direct aldol reactions between aldehydes (2-nitrobenzaldehyde, 2-methylpropanal) and acetone catalyzed by polystyrene resin (PS) supported di- and tripeptides H-Pro-Pro-, H-Pro-Pro-Pro-, H-Pro-Glu(OH)-, H-Pro-Pro-Glu(OH)-, H-Pro-Asp(OH)-, H-Pro-Pro-Asp(OH)-, H-Ser-Glu(OH)-, H-Ser-Ser-Glu(OH)-, H-Val-Glu(OH)-, H-Val-Val-Glu(OH)-MBHA-PS, were studied under identical experimental conditions at room temperature in a continuous-flow fixed-bed reactor (CFBR) system. In the asymmetric aldol reactions reversal of enantioselectivity was observed on H-Pro-Pro-Glu(OH)- and H-Pro-Pro-Asp(OH)-MBHA-PS-supported catalysts (ee 42–67% S) as compared to the H-Pro-Glu(OH)- and H-Pro-Asp(OH)-MBHA-PS-supported catalyst (ee 28–82% R). In the case of H-Pro-Pro- and H-Pro-Pro-Pro-MBHA-PS-supported catalysts reversed enantioselectivity was observed by using the benzoic acid additive (12% S) as compared to the H-Pro-MBHA-PS catalyst (25% R). The stability of the catalysts in the flow system was consistent with the heterogeneous character of the reaction, as was the linear behavior obtained using mixtures of L- and D-enantiomers of the supported H-Pro-MBHA-PS catalyst. The enamine character of the reaction intermediates was supported by ESI-MS measurements. Based on these and the computed structure of the peptides, the conformation of the intermediate adducts is held responsible for chiral induction, therefore for the enantioselectivity inversion observed in these reactions.

 Please wait while we load your content...
Please wait while we load your content...