Driving an equilibrium acetalization to completion in the presence of water†
Abstract
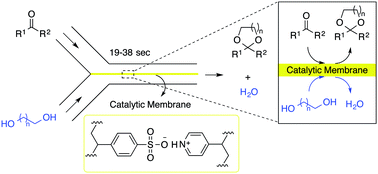
Formation of an acetal from a carbonyl substrate by condensation with an alcohol is a classical reversible equilibrium reaction in which the water formed must be removed to drive the reaction to completion. A new method has been developed for acetalization of carbonyl substrates by diols in the presence of water. Complexation of poly(4-styrenesulfonic acid) with poly(4-vinylpyridine) generates a catalytic membrane of polymeric acid at the interface between two parallel laminar flows in a microchannel of a microflow reactor. The catalytic membrane provides a permeable barrier between the organic layer and water-containing layer in the reaction, and permits discharge of water to the outlet of the microreactor to complete the acetalization. Condensation of a variety of carbonyl substrates with diols proceeded in the presence of water in the microflow device to give the corresponding acetals in yields of up to 97% for residence times of 19 to 38 s.

 Please wait while we load your content...
Please wait while we load your content...