Design, synthesis and preliminary evaluation of a novel SPECT DTPA-bis-triazaspirodecanone conjugate for D2 receptor imaging†
Abstract
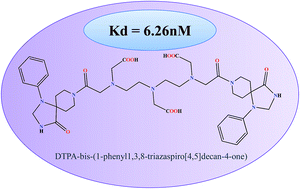
On the basis of pharmacological behaviour, dopamine receptors are divided into D1 and D2 subtype, which are primarily responsible for several neurophysiological anomalies. Assessment and validation with SPECT based conjugates provides a promising and cost effective insight to detect molecular changes in such neurological diseases. Spiperone, a well known “butryophenone” based D2 receptor antagonist, has been explored to design a novel conjugate for SPECT evaluation. The molecular docking pose analysis of the designed molecule was explored with dopamine D2 receptor. The molecule showed high affinity (−51.318 kcal mol−1) due to conserved interactions with Asp114 and Phe389 of D2 receptor. DTPA with triazaspirodecanone moiety of spiperone was synthesized using bifunctional chelation approach with 88% yield and has been characterized using NMR and mass spectroscopy. The radiolabeling efficiency of 99mTc-DTPA-bis-(1-phenyl-1,3,8-triazaspiro[4,5]decan-4-one) was 98%. The complex showed appreciable brain uptake in mice. Receptor binding experiments revealed a Kd of 6.26 nM with maximum localization of the conjugate in the striatum. Thus, these studies could be viewed as novel and informative, initial proof-of-concept approach to the field of 99mTc-labeled radioligand design.

 Please wait while we load your content...
Please wait while we load your content...