A facile access to highly functionalized triphenylphosphoranylidene succinimides through a three-component reaction and DFT investigation of the reaction mechanism†
Abstract
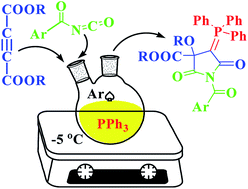
Novel, one-pot, three-component reaction of the zwitterions generated in situ from triphenylphosphine and acetylenic esters with aroyl isocyanate is described. The reaction afforded N-benzoyl-2-triphenylphosphoranylidene succinimide derivatives in good to high yields without using any catalyst or activation. Structural, electronic, energetic and mechanistic details of the reaction are also revealed by density functional theory (DFT) calculations, which strongly support the exclusive formation of the products.

 Please wait while we load your content...
Please wait while we load your content...