Preparation of an organic–inorganic polyurethane–Al2O3 anion exchange fibrous composite and its application in the development of a membrane electrode for the determination of chromium(vi) in water
Abstract
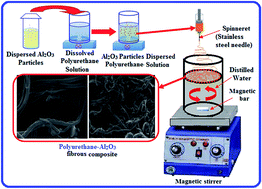
Polyurethane–Al2O3 organic–inorganic anion-exchange fibrous composites were prepared by the simple magnetic bar stirring of different stoichiometric ratios of polyurethane and Al2O3. The structures and morphologies of the prepared fibrous composites were ascertained by Fourier transform infrared spectroscopy (FTIR), field emission scanning electron microscopy (FE-SEM), thermal analyses (TGA and DTG) and X-ray diffraction (XRD). The material was found to be highly desirable for electroanalytical application as an ionic sensor, and its membrane electrode was developed for the detection of Cr(VI) in aqueous solutions. The membrane electrode shows the best result with a linear potential response in the concentration range of 1 × 10−1 mol L−1 to 1 × 10−8 mol L−1 of Cr(VI) ion with a slope of 30.36 mV per decade. The selectivity coefficients for interfering ions indicate good selectivity for Cr(VI) over interfering anions. The accuracy of the procedure was tested on chromium-free drinking water samples spiked with known amounts of Cr(VI), and the results were comparable to those obtained with AAS (atomic absorption spectroscopy).

 Please wait while we load your content...
Please wait while we load your content...