Basicity-controlled synthesis of Li4Ti5O12 nanocrystals by a solvothermal method†
Abstract
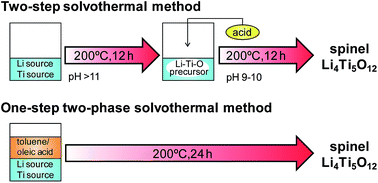
Spinel-type lithium titanate (Li4Ti5O12) nanocrystals were synthesized at a low temperature with control of the basicity by a solvothermal method without any calcination processes. The Li–Ti–O precursor was produced under relatively high pH conditions (pH > 11) in the initial stage. The spinel phase was then formed through delithiation and dehydration from the precursor by lowering the pH of the system (pH < 10). The direct synthesis of the Li4Ti5O12 nanocrystals was achieved through self-control of the basicity using a two-phase system of water–ethanol and toluene–oleic acid. The Li4Ti5O12 crystals were formed from the precursor by decreasing the basicity. The average size of the nanocrystals prepared by the one-step method was ca. 13 nm, and the specific surface area was 158 m2 g−1. On the basis of the electrochemical measurement, the Li4Ti5O12 nanocrystals produced in a low-temperature solution system are applicable for an anode material of a lithium-ion rechargeable battery.

 Please wait while we load your content...
Please wait while we load your content...