Cascade synthesis of 2-pyridones using acrylamides and ketones†
Abstract
Microwave assisted non-catalytic condensation of 2-cyanoacetamide with aromatic aldehydes, and enolate mediated Michael-type addition to acrylamide followed by oxidative cyclization, produce 2-pyridones in good to excellent yield. Unsymmetrical ketones produce two regioisomeric enolates, therefore thermodynamic and kinetic products of butan-2-one and pentan-2-one have been isolated and fully characterized.
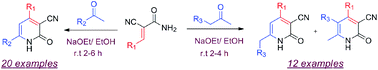

 Please wait while we load your content...
Please wait while we load your content...