Bipolar π-conjugation interrupted host polymers by metal-free superacid-catalyzed polymerization for single-layer electrophosphorescent diodes†
Abstract
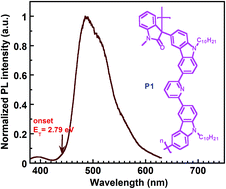
Novel aromatic bipolar host polymers (P1 and P2) containing pyridine as an electron transporting unit and carbazole and fluorene as hole transporting units in the π-conjugation interrupted polymer backbone have been synthesized by a metal-free superacid-catalyzed polyhydroxyalkylation. The present polymers show good thermal stability, with high glass transition temperatures and decomposition temperatures. The conjugation lengths of the polymers are effectively confined into the repeating units due to the δ-C bond interrupted polymer backbone, giving rise to quite high triplet energy (2.79 eV for P1) and a wide band gap of around 3.33 eV, which make them promising hosts for phosphorescent OLEDs. The results suggest that the strategy of incorporating bipolar blocks into the π-conjugation interrupted polymer backbone can be a promising approach to obtain host polymers with high triplet level for green and even blue phosphorescent polymer light-emitting diodes on a simple device structure and using a solution-processed technique.

 Please wait while we load your content...
Please wait while we load your content...