Nonstoichiometric calcium pyrophosphate: a highly efficient and selective catalyst for dehydration of lactic acid to acrylic acid†
Abstract
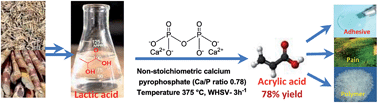
Calcium phosphate catalysts were prepared by co-precipitation method using calcium nitrate and mixtures of ammonium and different sodium phosphates as calcium and phosphate precursors, respectively. Depending on the phosphate precursor, the pH of the synthesis mixture changed during the catalyst precipitation. The catalyst characterisation by XRD and ICP revealed the formation of a calcium pyrophosphate structure with varying Ca/P ratio from 1.02 to 0.76 which could be correlated to the different pH of the synthesis solutions. Vapour phase dehydration of lactic acid to acrylic acid was carried out using these calcium pyrophosphate catalysts. Non-stoichiometric calcium pyrophosphate catalyst with Ca/P ratio 0.76 was found to be the most efficient catalyst among the synthesized series with 100% lactic acid conversion and 78% acrylic acid selectivity at 375 °C. The higher selectivity for acrylic acid has been correlated to the increased acidity and reduced basicity of non-stoichiometric calcium pyrophosphate compared to other stoichiometric pyrophosphates. In situ FTIR studies showed the formation of a higher amount of calcium lactate on non-stoichiometric compared to stoichiometric pyrophosphate leading to higher selectivity for acrylic acid.

 Please wait while we load your content...
Please wait while we load your content...