Amide-imide tautomerism of acetohydroxamic acid in aqueous solution: quantum calculation and SMD simulations†
Abstract
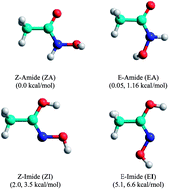
The kinetics and the thermodynamics of the amide-imide tautomerizations of acetohydroxamic acid in aqueous solution are studied from a theoretical point of view. Quantum electronic structure calculations (QCISD//MP2, MP2 and M06-2X, applying two continuum solvation methods) and steered molecular dynamic (SMD) simulations (with solute–solvent interaction potentials derived from AMBER van der Waals parameters and electrostatic charges in solution) are taken into account. The elementary and complex tautomerizations from the E-Amide and Z-Amide forms are studied with and without the explicit assistance of a water molecule. The Gibbs free energy of activation for the E-Amide ⇆ E-Imide and Z-Amide ⇆ Z-Imide processes are considerably reduced when these processes are assisted by one water molecule. The Gibbs free energy of reaction was also reduced, but to a lesser degree, for each of the processes considered when in the presence of an explicit solvent molecule. The Z-Amide ⇆ Z-Imide tautomerization, which occurs in two elementary steps, seems to be slightly more favoured from a thermodynamic point of view; however, the E-Amide ⇆ E-Imide tautomerization, which is an elementary process, is the most kinetically favoured. The SMD simulations led to results which are consistent with those obtained by the three electronic structure methods applied.

 Please wait while we load your content...
Please wait while we load your content...