Photophysical, bandstructural, and textural properties of o-FeNbO4 in relation to its cocatalyst-assisted photoactivity for water oxidation
Abstract
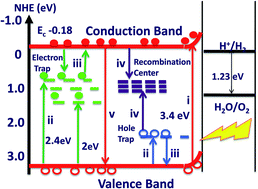
In this study, a relationship between physicochemical, photophysical and photocatalytic properties of hydrothermally synthesized orthorhombic iron niobate (FeNbO4) is investigated. o-FeNbO4 displayed a multi-regime optical absorbance, which was ascribed to at least two distinct phenomena: (i) bandgap (∼3.4 eV) excitation giving rise to UV absorbance and (ii) energy transitions involving disorder-induced sub-bandgap donor or acceptor states leading to visible light absorbance. The preparation-dependent distortion in the crystal lattice and the existence of closely spaced inter-bandgap energy states were corroborated by powder X-ray diffraction, photoluminescence, thermoluminescence, and Raman spectroscopy studies. The first principles electronic structure elucidation and photoelectrochemical measurements supported a wide bandgap for FeNbO4, in contrast to the narrow bandgap reported previously. Correspondingly, a small photocurrent density was observed for FeNbO4 (∼2 to 3 μA cm−2) under 1 sun illumination, suggesting the availability of a smaller cross section of photogenerated charge pairs. Following these band characteristics, while no H2 evolution was observed, FeNbO4 gave rise to particle size-dependent O2 evolution during visible light irradiation of water in the presence of electron scavengers, the samples loaded with NiO as cocatalyst showing better activity. Further, the transmission electron microscopy examination revealed the dominant exposure of (011) facets of FeNbO4, besides a significant heterogeneity of inter-domain boundaries. Overall, our results confirm that the photoactivity of metal/oxide nanocomposites is governed by a combination of factors, such as: grain morphology, microstructure, surface adsorption states, and the localized inter-bandgap energy states. Our study also reveals that, in contrast to prevalent assumptions, the wavelength at the absorption edge may not represent the true band-to-band energy gap of metal oxide semiconductors, which is relevant to their photocatalytic activity.

 Please wait while we load your content...
Please wait while we load your content...