Allylic amination of Passerini adducts. Application to the selective synthesis of chromone-substituted α-and γ-amino acid peptidic and retropeptidic units†
Abstract
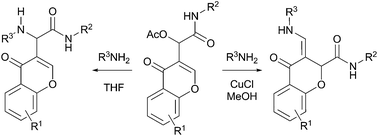
The first allylic amination of Passerini acetates is described, where both α and γ-substitution products are obtained regioselectively from chromone derived adducts. While α-substitution occurs in THF, CuCl in methanol catalyses a tandem amination followed by a rearrangement leading to γ-amino acid-derived chromones.

 Please wait while we load your content...
Please wait while we load your content...