Studies on electronic energy transfer (EET) on a series of room temperature ionic liquids (RTILs): can the EET studies on RTILs be exploited to predict their structural organization?†
Abstract
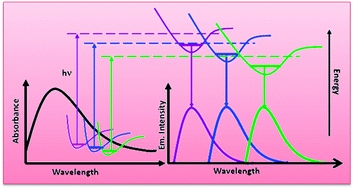
Steady state and time-resolved spectroscopic measurements have been carried out in two different series of neat ionic liquids where in one group (1-ethyl-3-methylimidazolium alkylsulfate) a systematic variation in alkyl chain length in their anionic moiety has been maintained keeping the cation fixed and in another series variation remains in the cation (morpholinium, pyrrolidinium and imidazolium) and anion (tris(pentafluoroethyl)trifluorophosphate) remains constant. The investigations have been carried out to get a better idea about the role of ionic constituents influencing different structural organisation in ILs. Even though observation of emission wavelength dependent decay behaviors of ILs may indicate the electronic energy transfer (EET) among different associated structures, absence of any rise-time during decay measurements certainly raises questions about the EET process. Emission wavelength dependent time-resolved data do not provide any straight forward linear correlation between these data and structural organisation of RTILs. Interestingly, significant fluorescence has also been observed even in neat non-aromatic RTILs resembling viscous glycerol.

 Please wait while we load your content...
Please wait while we load your content...