Primary arylamine-based tyrosine-targeted protein modification†
Abstract
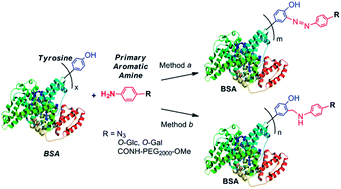
Tyrosine-targeted modification is of great interest in the site-specific protein modification applications. Aniline derivatives are attractive molecules for tyrosine-targeted protein modifications through either diazonium coupling or three-component Mannich type reactions. In this report, with BSA as a model protein, primary arylamines were demonstrated to incorporate bioorthogonal azide functionality for further site-specific protein modification via click chemistry, glycans for glyco-engineering, and PEG chains for PEGylation to the protein via tyrosine-targeted modification. The successful primary arylamine-based BSA modifications were confirmed by SDS-PAGE, western blot, and MALDI-TOF mass spectrometry. In comparison, three-component Mannich type reaction affords much higher reaction yields than diazonium coupling reaction in all modifications. Further, this study confirmed the importance of the electron withdrawing substituent on the para position of the phenyl ring for the tyrosine-targeted diazonium coupling reaction.

 Please wait while we load your content...
Please wait while we load your content...