Catalytic behavior of hydrogen radicals in the thermal decomposition of crystalline furoxan: DFT-based molecular dynamics simulations
Abstract
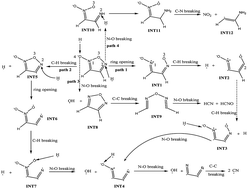
DFT-based MD simulations were performed to study the thermal decomposition of crystalline furoxan, an energetic nitrogen-containing model compound. The hydrogen radicals play a catalytic role in the following decomposition of furoxan and can promote the decomposition to a great degree. They not only catalyze the opening of furoxan rings and breaking of N–O bonds, but also capture and transport the oxygen atoms from nitrogen atoms to carbon atoms and promote the release of some small products. The catalytic ability may come from their high mobility that enables the hydrogen to transport fast between different species.

 Please wait while we load your content...
Please wait while we load your content...