Site activation effects promoted by intramolecular hydrogen bond interactions in SNAr reactions†
Abstract
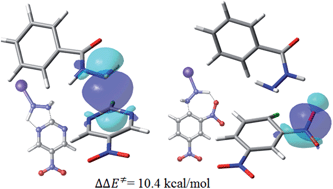
The nucleophilic aromatic substitution reaction of benzohydrazide derivatives towards 2-chloro-5-nitropyrimidine is used as model system to experimentally and theoretically show that intramolecular hydrogen-bond formation operates as a perturbation that elicits a dual response at the reaction center of the transition state (TS) structure, by enhancing the electrophilicity of the pyrimidine moiety and the nucleophilicity of the nitrogen atom of the benzohydrazide fragment. The electronic mechanism can therefore be described as a (non-local) site activation problem.

 Please wait while we load your content...
Please wait while we load your content...