Hydrogen physisorption in ionic solid compounds with exposed metal cations at room temperature†
Abstract
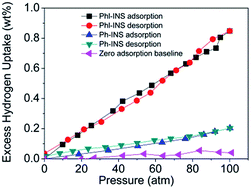
Phenol- and phloroglucinol-based organo-magnesium ionic solid compounds were synthesized for room temperature hydrogen storage via physisorption. These materials contain magnesium dications balanced with highly charge-delocalized anionic species, making the cations highly exposed with relatively weak electrostatic interactions with the anions, and thus facilitates an interaction with molecular hydrogen. It was found that the driving force for the hydrogen physisorption in these materials is largely electrostatic with the σ electrons of hydrogen partially polarized by the cationic charges. The synthesized amorphous complexes exhibit moderate surface areas up to 165 m2 g−1 and 115 m2 g−1 with maximum excess hydrogen sorption capacities of 0.22 wt% and 0.8 wt%, respectively, at 298 K and 100 atm. The isosteric heat of adsorption was calculated from Clausius-Clapeyron equation using isotherms at 323 K, 298 K and 273 K. The results confirm low coverage isosteric heat of adsorption of 7.2 kJ mol−1 and 12 kJ mol−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...