Base-catalyzed retro-Claisen condensation: a convenient esterification of alcohols via C–C bond cleavage of ketones to afford acylating sources†
Abstract
The base-catalyzed esterification of alcohols via retro-Claisen condensation has been demonstrated for the first time. A variety of alcohols including aryl- and heteroaryl methanols as well as aliphatic ones underwent efficient acylation to give the ester products in reasonable to good yields upon isolation. Not only conventional 1,3-diketones but also strong electron-withdrawing group containing acetophenones could serve as the acylating suppliers. The synthetic protocol is operationally simple and adaptable to a broad substrate scope. It complements the existed esterification via the retro-Claisen condensation, and enlarges this benign synthetic methodology.
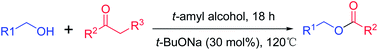

 Please wait while we load your content...
Please wait while we load your content...