A novel, concise and efficient protocol for non-natural piperidine compounds†
Abstract
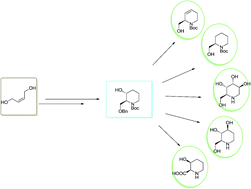
Formal synthesis of L-altro-1-deoxynojirimycin, cis-3-hydroxypipecolic acid along with synthesis of (R)-piperidinol and a conceptually different advanced intermediate for non-natural piperidine alkaloids is reported from cis-butene-1,4-diol. The key reactions involved are Johnson–Claisen rearrangement, Sharpless asymmetric dihydroxylation, reductive lactamization and novel regioselective elimination.

 Please wait while we load your content...
Please wait while we load your content...