Multifarious zinc coordination polymers based on biphenyl-3,3′,5,5′-tetracarboxylate and different flexibility of N-donor ligands†
Abstract
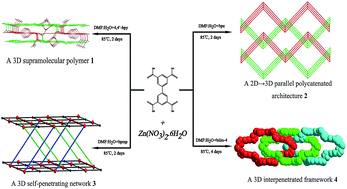
Based on different flexibility of N-donor and biphenyl-3,3′,5,5′-tetracarboxylate ligands we successfully construct four novel Zn(II) coordination polymers, formulated as {[Zn2(bptc)(4,4′-bpy) (H2O)2]·2H2O}n (1), {[Zn(H2bptc)(bpe)]·2H2O}n (2), {[Zn2(bptc)(bpmp)2]·2H2O}n (3) and {[Zn2(bptc)(biim-4)2]·H2O}n (4) [H4bptc = biphenyl-3,3′,5,5′-tetracarboxylic acid, 4,4′-bpy = 4,4′-bipyridine, bpe = 1,2-bis(4-pyridyl)ethane, bpmp = N,N′-bis-(4-pyridyl-methyl) piperazine and biim-4 = 1,1′-(1,4-butanediyl)bis(imidazole)]. Single-crystal X-ray diffraction studies reveal that these four coordination polymers present various topological supramolecular architectures originated from auxiliary N-donor ligands with different flexibility. Complex 1 displays a 3D supramolecular polymer based on the rigid 4,4′-bpy ligands. Complex 2 shows a twofold 2D → 3D framework by parallel polycatenation of undulating 2D sheets based on the semi-rigid bpe ligands. Complex 3 exhibits an unusual 3D self-penetrating coordination framework constructed from the semi-rigid bpmp ligands. Complex 4 is a unique threefold 3D interpenetrated framework based on the flexible biim-4 ligands. In addition, their solid-state luminescent properties also have been systematically investigated.

 Please wait while we load your content...
Please wait while we load your content...