One-step synthesis of noble metal/oxide nanocomposites with tunable size of noble metal particles and their size-dependent catalytic activity†
Abstract
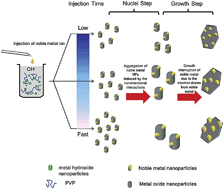
Synthesis of noble metal/oxide nanocomposites by a facile method is still essential and much needed. This study demonstrates a general one-step strategy for the synthesis of noble metal/oxide nanocomposites by an aqueous route with reaction between the metal hydroxide and noble metal ion. Pd–CeO2, Au–Fe3O4 and Ag–Mn3O4 nanocomposites were fabricated as examples, revealing the generality of the synthesis method. The noble metal nanoparticles are tunable in size achieved through adjustment of the injection rate of the solution containing the noble metal ions rather than by changing the loading of noble metal. We propose a mechanism for the size variation of the noble metal nanoparticles with the injection rate of the noble metal ion solution. The as-synthesized nanocomposites have a stable structure and high yield (>98%) of noble metal. Both the synthesis and size-control methods can be extended to the preparation of other nanocomposites. In addition, all of the as-synthesized nanocomposites exhibit superior catalytic activities for hydrogenation reactions in aqueous solution, dependent on the size of the active metal particle.

 Please wait while we load your content...
Please wait while we load your content...