Effectiveness of electrochemical degradation of sulfamethazine on a nanocomposite SnO2 electrode
Abstract
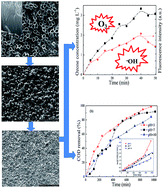
A nanocomposite SnO2 electrode was prepared and its activity on electrocatalytic degradation of sulfamethazine was investigated. Compared with the traditionally dip-coated SnO2 electrode and the dip-coated SnO2 on TiO2 nanotube electrode, the nanocomposite SnO2 electrode has better surface configuration, better crystallinity and a higher electrocatalyst loading, and it also shows better electrocatalytic activity on sulfamethazine degradation. The sulfamethazine elimination rate on the nanocomposite SnO2 electrode increases with current density in the range of 10–30 mA cm−2, but decreases when the current density further increases to 50 mA cm−2. The chemical oxygen demand (COD) removal rate increases with current density in the range of 10–50 mA cm−2, whereas the increase of current density results in a decrease of current efficiency. The solution pH has a certain effect on sulfamethazine degradation as an increase of pH from 3.0 to 10.0 can lower both the sulfamethazine elimination rate and the COD removal rate. Ozone generation and hydroxyl radical formation are determined in the electrolytic system. The oxygen evolution potential of the nanocomposite SnO2 electrode decreases with the increase of solution pH, which should be responsible for the better performance of this electrode on sulfamethazine degradation in acidic electrolyte.

 Please wait while we load your content...
Please wait while we load your content...