Bis(oxazoline)-copper catalyzed enantioselective hydrophosphonylation of aldehydes†
Abstract
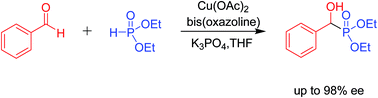
A highly enantioselective copper-catalyzed hydrophosphonylation of aldehydes in the presence of bis(oxazoline) ligand is presented. The reaction proceeded smoothly under mild conditions. The resulting α-hydroxyphosphonates were obtained with high yields as well as enantioselectivities (up to 98% ee).

 Please wait while we load your content...
Please wait while we load your content...